As pioneers in the field of electronic connector solutions, companies like Shenzhen Haiyuncheng Electronic Co., Ltd. are at the forefront of innovation. Specializing in connector design, development, production, and sales, they possess cutting-edge capabilities such as injection molding, terminal stamping, mold design, and a suite of imported precision measuring instruments. These advanced technologies and processes are crucial for manufacturing both IDC and crimp connectors, ensuring that they meet the highest standards of quality and performance.
Electrical connectors are the unsung heroes of modern electronics, ensuring reliable signal and power transmission across countless devices and systems. Among the various connection technologies available, Insulation Displacement Connection (IDC) and crimp connectors stand out as two widely used methods, each with distinct characteristics that make them suitable for different applications.
The primary difference between IDC and crimp connectors lies in their connection mechanisms: IDC connectors pierce through wire insulation to establish contact without stripping, while crimp connectors require stripped wires and use mechanical compression to create a gas-tight connection between terminal and conductor. Both methods eliminate the need for soldering but achieve this through fundamentally different approaches.
Understanding these differences is crucial for engineers, technicians, and procurement specialists working with electrical systems. The choice between IDC and crimp technology can significantly impact product reliability, manufacturing efficiency, and long-term performance in various environmental conditions.
This comprehensive guide will explore:
1. Fundamental working principles of both connection types
2. Mechanical and electrical performance comparisons
3. Application-specific advantages and limitations
4. Reliability considerations for different environments
5. Cost and production efficiency factors
How IDC and Crimp Connectors Work
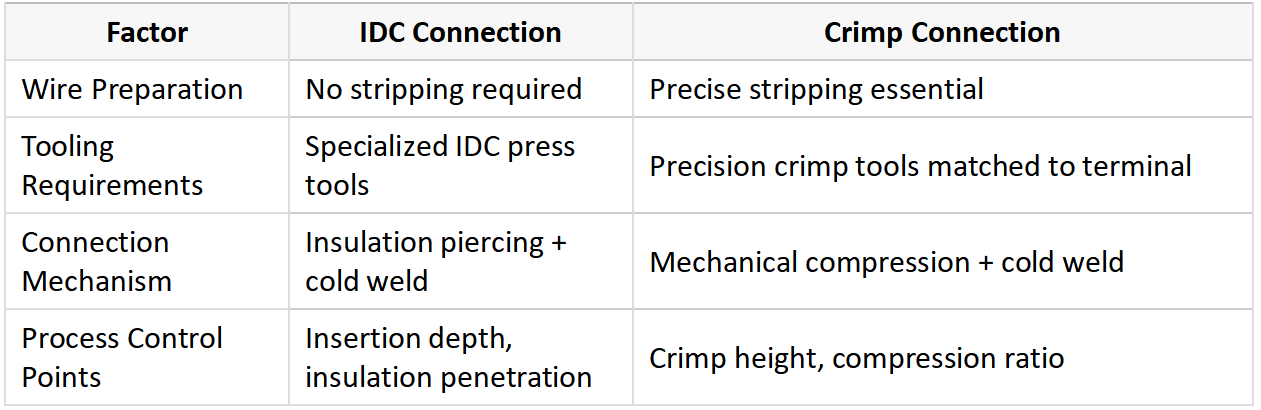
IDC connectors establish electrical contact by forcing a insulated wire into a V-shaped metal slot that cuts through the insulation and makes direct contact with the conductor, while crimp connectors require pre-stripped wires that are mechanically compressed within a metal terminal to form a cold-welded joint.
The insulation displacement technology behind IDC connectors represents a clever solution for mass termination applications. When a wire is pressed into the connector’s V-shaped slot, the sharp edges penetrate the insulation and establish contact with the conductor through a cold-welding process. This method eliminates the need for separate stripping operations, significantly reducing assembly time. The connection quality depends on several factors including insulation hardness, conductor cross-section, and the precision of the terminal geometry. Modern IDC connectors often incorporate strain relief mechanisms to prevent wire pull-out in vibrating environments.
Crimp technology operates on different principles. The process involves inserting a stripped wire into a metal terminal (either machined or stamped) and applying precise pressure to deform both components. This plastic deformation creates a gas-tight interface that prevents oxygen penetration and subsequent oxidation. The crimping process must exceed the wire’s yield point to ensure proper metal flow and permanent deformation. Quality crimp connections show consistent cross-sections when viewed under microscopy, with the conductor strands uniformly compressed and the terminal material flowing around them.
Key process differences:
Performance Comparison: Electrical and Mechanical Characteristics
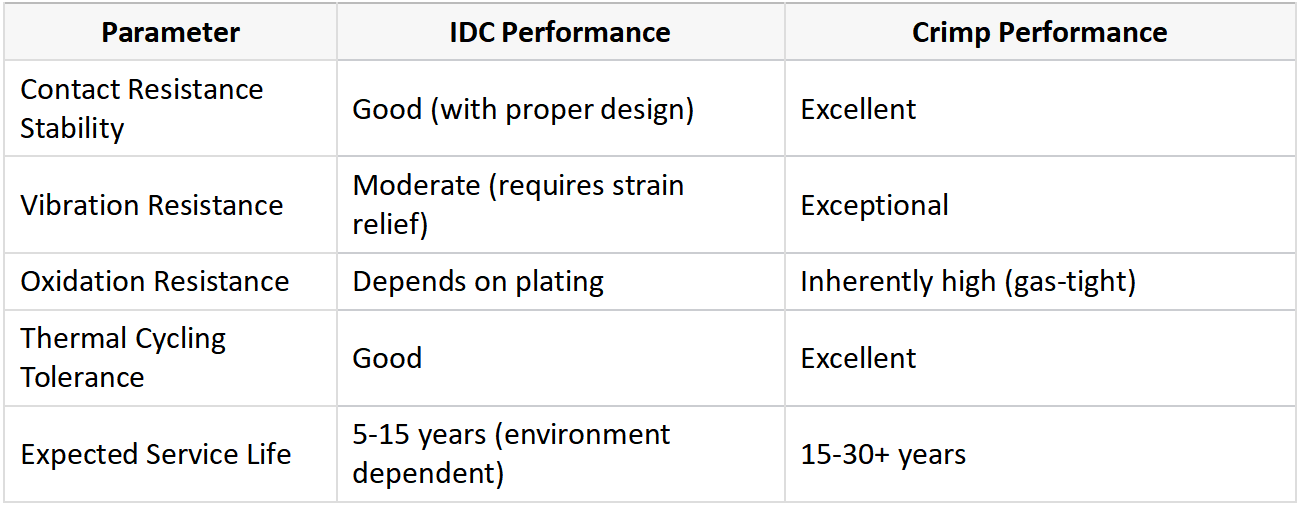
IDC connectors typically exhibit higher contact resistance but are sufficient for signal applications, while crimp connections offer superior conductivity and mechanical strength, making them ideal for power transmission and harsh environments.
Electrically, crimp connections generally outperform IDC in several key metrics. The cold-welded interface of a properly executed crimp creates a metallurgical bond with contact resistance often below 1 milliohm. This makes crimp connections particularly suitable for power applications where current carrying capacity and minimal voltage drop are critical. IDC connections, while electrically adequate for many applications, inherently have higher resistance due to smaller contact areas and potential insulation remnants at the contact interface. However, high-quality IDC designs can achieve stable contact resistance when properly implemented, especially in low-current signal applications.
Mechanically, crimp connections demonstrate superior vibration resistance and wire retention. Automotive and aerospace applications frequently specify crimp connections because they can withstand constant vibration without degradation. The complete encapsulation of conductor strands within the crimp terminal provides excellent strain relief. IDC connections rely more on the insulation clamping force and supplemental strain relief features, which may be insufficient for high-vibration applications without additional securing methods.
Environmental performance differs significantly:
1. Temperature resistance: Crimp connections typically handle wider temperature ranges due to their metallic interfaces, while IDC performance may degrade at extremes
2. Corrosion resistance: Both benefit from plating (tin, gold, silver), but crimp’s gas-tight seal provides better long-term protection
3. Moisture resistance: IDC’s insulation penetration creates potential moisture ingress points absent in crimp connections
4. Thermal cycling: Crimp connections better accommodate conductor/terminal differential expansion
Application-Specific Advantages and Limitations
IDC connectors excel in high-density, rapid-assembly applications like ribbon cable connections, while crimp technology dominates in reliability-critical applications such as automotive wiring and aerospace systems.
The electronics industry has adopted IDC technology extensively for flat ribbon cable connections, particularly in computer and telecommunications equipment. The ability to terminate multiple conductors simultaneously makes IDC ideal for mass termination applications. Modern IDC connectors support conductor sizes from 28 AWG to 14 AWG, accommodating both solid and stranded wires in many cases. Their compact form factor benefits space-constrained designs, and the elimination of stripping operations reduces production time and cost for high-volume manufacturing.
Crimp connections find their strongest applications where reliability cannot be compromised. Automotive wiring harnesses, aircraft systems, and industrial equipment overwhelmingly use crimp technology for its vibration resistance and long-term stability. The technology supports an extremely wide range of conductor sizes (from 0.08mm² to 240mm²) and can accommodate both fine-stranded and solid conductors with appropriate tooling. Crimp terminals also facilitate modular wiring systems, allowing field repairs and modifications that would be impossible with IDC connections.
Industry-specific preferences:
- utomotive: 90% crimp due to vibration requirements; some IDC in interior electronics
- elecommunications: Mixed use – IDC for backplane connections, crimp for field-terminated cables
- onsumer Electronics: Predominantly IDC for internal ribbon cables
- ndustrial Controls: Mostly crimp for reliability; some IDC in control panels
- erospace: Exclusively crimp for maximum reliability
Reliability and Long-Term Performance Factors
Crimp connections generally offer superior long-term reliability due to their gas-tight seal and robust mechanical retention, while IDC connections may degrade over time in challenging environments without proper design precautions.
The gas-tight interface created by proper crimping prevents oxygen penetration, virtually eliminating oxidative degradation at the contact point. This characteristic, combined with the metallurgical bond formed during compression, gives crimp connections exceptional longevity even in harsh environments. Industrial studies have shown properly executed crimp connections maintaining stable resistance for decades in field applications. The mechanical interlock between terminal and conductor also resists vibration-induced loosening, a critical factor in transportation applications.
IDC connections face more reliability challenges due to their fundamental design. The insulation displacement process can leave microscopic gaps where moisture and contaminants may ingress over time. While high-quality IDC connectors incorporate features to mitigate these issues (such as corrosion-resistant plating and insulation-gripping designs), they remain inherently more vulnerable to environmental factors than crimp connections. However, in controlled environments (indoor electronics, sealed enclosures), IDC reliability can approach that of crimp connections for the operational life of most electronic products.
Key reliability indicators:
Production Efficiency and Cost Considerations
IDC technology provides significant cost and time savings in high-volume production environments, while crimp connections offer better field-serviceability and flexibility for low-volume or customized wiring applications.
The economic advantages of IDC become apparent in mass production scenarios. A single IDC termination operation can simultaneously connect multiple conductors in a ribbon cable, achieving termination rates impossible with individual crimps. The elimination of wire stripping further reduces production steps and associated costs. For standardized connections in consumer electronics and office equipment, IDC often delivers the lowest per-connection cost. The technology also requires less operator skill compared to crimping, reducing training costs and quality variability in high-turnover production environments.
Crimp technology, while generally more labor-intensive, offers compensating advantages in flexibility and reparability. The same basic crimp tools can handle various wire sizes and terminal types, making the technology ideal for customized or low-volume production. Field installations and repairs favor crimp connections because they don’t require specialized equipment beyond hand tools. The modular nature of crimp terminals also simplifies inventory management compared to the numerous IDC connector variants needed for different applications.
Cost factor comparison:
1. Tooling Investment: IDC requires dedicated dies for each connector type; crimp tools are more universal
2. Labor Costs: IDC typically lower for high-volume; crimp more efficient for small batches
3. Material Costs: IDC connectors often cheaper; crimp terminals vary widely by quality
4. Rework Costs: IDC connections are difficult to rework; crimp connections easily replaced
5. Quality Control: Both require process control, but crimp parameters are more easily verified
Conclusion
The choice between IDC and crimp connection technologies involves careful consideration of electrical requirements, mechanical stresses, environmental conditions, and production realities. IDC technology shines in high-density, high-volume applications where rapid installation and cost efficiency outweigh ultimate reliability needs. Crimp technology remains the gold standard for mission-critical applications where vibration resistance, long-term stability, and field serviceability are paramount.
Modern engineering often employs both technologies within the same system – using IDC for internal board-to-board connections while relying on crimp for external wiring and power distribution. As connection technologies evolve, hybrid approaches are emerging that combine the best features of both methods, promising even greater reliability and efficiency for future electronic systems.
Understanding these fundamental connection technologies empowers design engineers to make informed decisions that optimize both product performance and manufacturing efficiency. Whether specifying IDC for a consumer electronics assembly or crimp terminals for an aerospace harness, the principles discussed here provide a solid foundation for connection technology selection.
Media Contact
Company Name:Shenzhen Haiyuncheng Electronic Co., Ltd.
City, State, Country:Shenzhen City, Guangdong Province, China.
Address: A building,Baitong science innovation park, No. 150 Shasong RD., Shajing Sub-district
E-mail:sales04@hrb-connector.com
Contact Person: Doria
Website: https://www.hrb-connector.com/